Anticancer Effects of Grape Seed Extract on Human Cancers: A Review

Dinicola S1, Cucina A2, Antonacci D3 and Bizzarri M4*
1Department of Clinical and Molecular Medicine, La Sapienza University, Rome, Italy
2Department of Surgery ‘‘Pietro Valdoni,’’ La Sapienza University, Rome, Italy
3Consiglio per la ricerca e la sperimentazione in agricoltura, Unità di ricerca UTV, Turi BA, Italy
4Department of Experimental Medicine, La Sapienza University, Rome, Italy
- *Corresponding Author:
- Mariano Bizzarri
Department of Experimental Medicine
La Sapienza University, Rome, Italy
Tel: +39(0)649766606
Fax: +39(0)649766897
E-mail: [email protected]
Received date: November 15, 2013; Accepted date: December 31, 2013; Published date: January 06, 2014
Citation: Dinicola S, Cucina A, Antonacci D, Bizzarri M (2014) Anticancer Effects of Grape Seed Extract on Human Cancers: A Review. J Carcinog Mutagen S8:005. doi: 10.4172/2157-2518.S8-005
Copyright: © 2014 Dinicola S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Carcinogenesis & Mutagenesis
Abstract
Grape seed extract (GSE) is a complex mixture of several compounds, mostly represented by polyphenols and phenolic acids. Their consumption is safe and is recognized to exert several and meaningful health benefits. In particular, grape-related anti-tumoral activity encompasses a wide array of biological mechanisms and cellular targets, eventually leading to inhibition of cell growth and to enhanced apoptosis in several cancer cell lines, including lung, colon, breast, bladder, leukemia and prostate tumors. Those effects are likely modulated at the molecular level through selectively modulating the redox balance and displaying anti-oxidant as well as pro-oxidant actions, according to the specific context. GSE-related anti-cancer activity mostly relies on the induced increase in reactive oxygen species, followed by the orchestrated down- and up-regulation of several key-molecular pathways, including MAPK kinases, PI3K/Akt, NF-kB, cytoskeleton proteins and metalloproteinases. Promising results obtained in vitro as well as on animal studies suggest that GSE may have a great relevance as source of potential new pharmacological molecules, and could represent an important opportunity for clinical research.
Keywords
GSE; Apoptosis; Chemoprevention; Oxidation
Introduction
Grape seeds and fruits
Cancer is among the leading cause of death in the Western world and its incidence is rising sharply in the developing countries too. By no doubt, that trend can be likely ascribed to the world-wide adoption of western dietary habits, characterized by high saturated fat diet, low intake of fresh vegetables and fruits, with reduced assumption of polyphenolic-rich foods (like green tea, soy and grape seeds) [1]. On the contrary, high and regular consumption of polyphenolic-rich foods has proven to significantly reduce the incidence of breast, lung, prostate and gastro-intestinal human cancers [2]. Among those foods, a prominent role is undoubtedly sustained by grapes and grape-related aliment and beverages.
From time immemorial grapes have been used both for medicinal and nourishment purposes, chiefly in Greece and in Italy. Grapes (Vitis vinifera) have been heralded for their medicinal and nutritional value for thousands of years: Egyptians ate grapes at least 6,000 years ago, and several ancient Greek philosophers praised the healing power of grapes, usually in the form of wine. The role that the grape has in the food culture of the Mediterranean countries is comparable only to that played by tea in among the peoples of Asia, indeed. An impressive body of the current scientific literature supports the health benefits claimed by the medical tradition. Several epidemiological studies have associated the consumption of grapes, wine, and grape juice with a wide variety of health-promoting effects, particularly the reduced risk of cancer and cardiovascular diseases [3–6]. It is worth of mentioning that a significant linear decrease in risk of lung cancer associated with consumption of red wine among eversmokers has been recorded by a multiethnic cohort study involving more than 80,000 men: consumption of 1-2 cup of wine reduces the risk of lung cancer of approximately 60%. A similar trend has been observed by other studies [7–10]. Interestingly, a similar pattern has been recorded by epidemiological studies performed on Green Tea [11–14].
Tea and grape have different chemical composition [15]. Yet, many GSE components (epigallo-catechins, procyanidins, flavonoids) are also found in Green Tea, and they may well account for the widely recognized beneficial effects of tea consumption. However, even if a consistent overlap has been observed in between the biological properties of both mixtures, yet extracts from grapes and tea differ significantly in their effectiveness, given that when they are simultaneously added to cancer cells, a synergistic, significant effect can be observed [16]. Yet, the beneficial properties of both tea and grape (or grape derived food products), are believed to be related to their polyphenolic content [17,18]; and, by no doubt, grapes constitute one of the major sources of phenolic compounds among fruits [19].
Grape seed composition
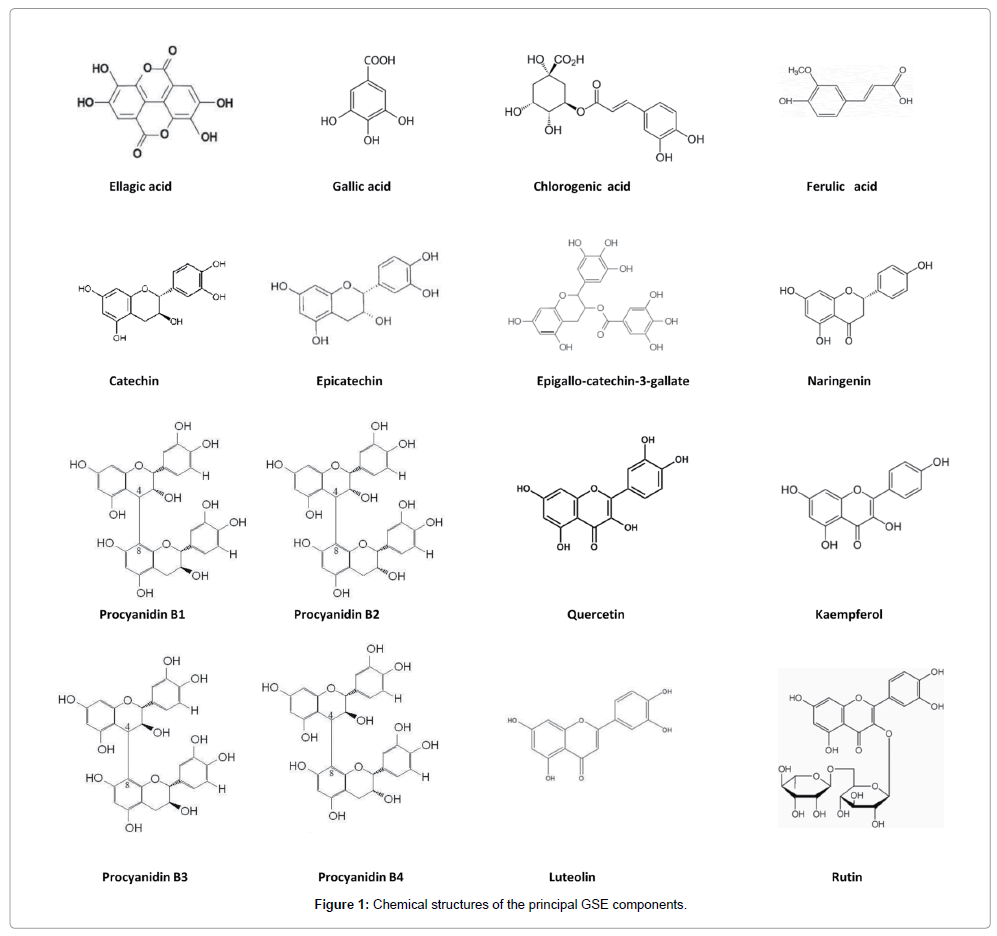
Grape seed composition differs significantly in between different cultivars [20–23], namely when white versus red grapes are considered. Yet, those differences reflect not only genetic variability, but also highlight the impact of vineyard treatments, ripeness grade [24,25], irrigation strategy [26,27] and nitrogen fertilization [28]. Even within seeds obtained from the same cultivar a significant variability in chemical composition has been recorded, and such a result may be likely ascribed to differences in the extraction method [29–31]. In addition, several environmental and biological factors, such as hyperopic, light, drought, high salinity, cold, metal ions, pollutants, xenobiotics, toxins, reoxygenation after anoxia, experimental manipulations, pathogenic infection and ageing of plants may affect yields and seed quality, mainly by inducing oxidative stress [32,33]. Nonetheless, plant cells have a wide array of detoxifying enzymes and pharmacologically active, anti-oxidant compounds that scavenge Reactive Oxygen Species (ROS), participate in seed survival, and may hence display relevant pharmacological activities [34]. Besides some minor components, main grape seed constituents are represented by polyphenols, phenolic and hydroxy-benzoic acids. Stylbenes (trans-resveratrol) as well, are occasionally found, even if in a few varieties [35]. Polyphenols (Flavonoids) is a collective noun given to several classes of structurally similar compounds, having a common C6-C3-C6 flavone skeleton in which the three-carbon bridge between the phenyl groups is commonly cyclized with oxygen. Flavonoids include several classes of compounds: Flavones (luteolin), Flavan-3-ols (catechins, epicatechins, epigallo-catechins, epigallocatechin-3-gallate, procyanidins), Flavanones (neringein), and Flavonols (quercetin, rutin, kaempherol) and Anthocyanins (Table 1) [36]. Each class differs from the other according to the degree of unsaturation and oxidation of the three-carbon segment [37]. Flavonoids are usually present in nature as glycosides: the sugar moiety attached to the flavonoid structure affects ease of absorption from the intestinal tract and the bioavailability of the compound. Yet, glycosylation lessens the reactivity of flavonoids against free radicals and slow-down their intestinal absorption [38]. Grape seeds have higher content of both phenolic acids and flavonoids (where they account for 60-70% of dry extract) [39] than grape skin and whole grape extract, meanwhile resveratrol and anthocyanidins are more abundant in the latter two extracts [40].
| Hydroxybenzoic and Phenolic Aids | Polyphenols (Flavonoids) | Still Benes | |||||
| Ellagic acid* | Flavones Luteolin* Diosmetin Apigenin Chrysin wogonin | Flava-3-ols Catechin* Edpicatechin* Epigalloctechin* Epigalloctechin-3-gallete* B1,B2,B3,B4 procyanidins* | Flavanones Naringenin* Hesperidin Eriodictyol | Flavonds Quercetin* Myricetin* Kaemoferol* Rutin* | Isoflavones Genistein Daidzein | Anthocyamins Delphinidin Pelargonidin malvidin cyanidin petunidin | |
| Gallic acid* | Reveratrol* | ||||||
| Vanillic acid* | Trans-reveratrol* | ||||||
| Caffeic acid* | |||||||
| Coumaric acid* | |||||||
| Ferulic acid* | |||||||
| Gentistic acid* | |||||||
Table 1: Grape seed composition. Principal classes of polyphenols and phenolic acids; stars evidence compounds found in grape seeds by different analytical studies [15,19-22,28-30,39-40].
Several individual grape seed components (Figure 1) have been demonstrated to display relevant chemical and biological functions, such as antioxidant [41], anti-inflammatory [42], inhibition of platelet aggregation [43], antimicrobial [44], and “anti-aging” activities [45]. Those properties have been found to be directly associated to the total polyphenolic content [46,47] and specifically ascribed to the activity of the more effective components, among which ellagic [48] and gallic acid [49], epigallocatechin-3-gallate [50], procyanidins [51] and quercetin [52] are by far the most important. Gallic acid, procyanidins and epigallo-catechins overall account for about 80-90% of dry extract [53], and medical properties of grape seeds are generally referred to those molecules, indeed [54]. Yet, the contribution of other active, even less represented molecules cannot be excluded, given that some biological functions seem to be synergistically afforded by interactions among the different components [55]. Namely, the well-known anti-oxidant effects exerted by GSE, can only barely be explained by the sum of the anti-oxidant activities of each individual component. Indeed, correlation analysis showed that none of the identified polyphenols had a strong correlation with protection from ROS [56]. Thus, it seems that there may be a synergism between polyphenols and/ or between polyphenols and phenolic acids and other phytochemicals. Similarly, even if anticancer effects are generally thought to be exerted mainly by procyanidins and epigallo-catechin-3-gallate (EGCG), again the overall GSE anticancer effect is higher than that obtained by the sum of each individual component [57].
Dual Effects of Grape Seed Extract: Anti-Oxidant and Pro- Oxidant Activities
GSE, as well as many of its individual components have demonstrated both in vitro and in vivo to prevent carcinogenesis [58–60], to inhibit cancer cell proliferation and to enhance cancer cells apoptosis, often reaching efficiency rates equal or greater than that achieved by conventional drugs. Yet, the by far most relevant feature of GSE is the dual role displayed in normal and cancerous cells. Grape and tea extract are safe, even at the highest concentrations [61–63], and exert a wide array of protective actions. Indeed, GSE and many phytochemicals inhibit apoptotic process and display a strong anti-oxidant effect on normal cells, meanwhile the opposite is true for cancer cells: neither growth inhibition nor apoptosis have been noticed in normal cells even at higher doses of GSE [64]. How and why that paradoxical behaviour occur is still a matter of investigation, even if an increasing body of evidence suggest that a possible explanation may be provided by the dual role exerted on the intracellular ROS formation.
GSE displays pro-oxidant effects on cancer cells
Antioxidant activities of GSE and grape phenolic compounds (mainly resveratrol and procyanidins), have been extensively investigated in vitro and in vivo [65]. GSE possesses strong free radical scavenging activity [66], prevents ROS-induced DNA damage [67], and displays a relevant chelating effect on transition metal ions, thus reducing lipid peroxidation [68]. Those effects have been deemed even more potent than known antioxidants such as vitamin E and ascorbic acid [69]. Some studies have reported an enhancing effect of GSE or of its polyphenolic constituents, on several antioxidant enzymes as glutathione (GSH) [70], super-oxide dismutase (SOD) [71], catalase [72] and other detoxifying/antioxidant enzymes [73]. GSEinduced antioxidant enzyme expression is associated with activation of the redox-sensitive transcription factor nuclear factor erythroid-2 p45 (NFE2)- related factor (Nrf2), through its interaction with the antioxidantresponse element (ARE) or the electrophile-responsive element (EpRE) [74,75]. Indeed, Nrf2 plays a key role in up-regulation of many phase II antioxidant/detoxifying enzymes, including glutathione peroxidase (GPx), glutamate cysteine ligase (GCL), glutathione S-transferase (GST), SOD, and NADPH/quinone oxidoreductase 1 (NQO1) [76].
In vivo, dietary supplementation of GSE was shown to reduce oxidative stress and improve the glutathione/oxidized glutathione ratio, as well as the total antioxidant in a double-blinded randomized crossover human trial [77]. Though those results have been often confirmed [78], other studies have been unable to do so, showing that GSE exhibits either only a moderate or negligible antioxidant effect [79,80].
Oxidative stress, resulting from enhanced production of ROS overcoming the cellular antioxidant defence, is a key phenomenon in chronic degenerative diseases (diabetes mellilitus, cardiovascular illness, cancer) [81,82]. ROS participate in triggering the apoptotic process, as programmed cell death is tightly regulated by the oxidative environment [83]. Dietary GSE strongly reduces rat mucosal apoptosis via modulation of both mitochondrial and cytosolic antioxidant enzyme systems together with an increase in cellular GSH, thus protecting normal colonic mucosa from ROS injury [58,84]. Given that GSE exerts a protective antioxidant effect in normal cells exhibiting deficiency of catalase activity or glutathione level, it can be hypothesized that grape polyphenols participate in controlling intracellular peroxide production [85]. Hence, anti-oxidant properties of GSE treatment may efficiently counteract the onset of ROSdependent disease, as documented by several studies [86]. Yet, despite the popular version diffused by mass-media, it is hardly conceivable that GSE or polyphenols may exert a significant effect against cancer development by displaying anti-oxidant actions [87].
Indeed, several studies have reported that GSE in cancer cells paradoxically enhances ROS production in a significant manner. GSE and many polyphenolic compounds induce a relevant increase in ROS and in superoxide radical generation, at both the cytosolic and mitochondrial site, that could eventually lead to GSH depletion [88]. It is worth noting that GSE does not induce hydroxy peroxide (H2O2) increase, thus evidencing a deficiency in SOD activity, at least in the cancer cell lines studied. Indeed, in SOD-deficient cells, GSE treatment induce ROS-mediated cytotoxicity, evidencing that GSE-dependent increase in ROS activity is not efficiently counteracted by SOD-dependent transformation in hydroxy peroxide, leading to GSH depletion, cellular damage, and increased apoptosis [89]. Moreover, pro-oxidant effects of GSE are enhanced in cells lacking SOD activity [90], meanwhile co-exposures of polyphenols-treated cancer cells with SOD largely prevented ROS formation and DNA damage [91]. Considering that the oxidant-dependent toxicity of polyphenols is efficiently rescued by co-treatment with SOD, but not with catalase, it is unlikely that flavonoids-related pro-oxidant effects could be mediated by H2O2, but rather by the super oxide anion O2·– , whose activity is unaffected by catalase, indeed [92]. This specific feature is displayed by the overall extract from grape seed, as pro-oxidant effects triggered by individual polyphenols – obtained from tea or grapes – can be significantly inhibited by adding catalase in the culture medium [93,94]. Several indirect experimental evidences support these observations. Indeed, cell culture medium amended with either green tea or with red wine inhibited proliferation of rat pheochromocytoma PC12 cells: both supplementation generated ROS, and the addition of catalase completely abolished the antiproliferative effects of green tea, but only partially reduced that of red wine [95]. Apparently, this result would suggest that ROS accounted for the total cytotoxic effect of green tea, but only partially for that of red wine. As catalase may detoxify only H2O2, it should be hypothesized that anticancer actions of GSE may be exerted through the increase of different reactive oxygen species, among which H2O2 represent only a negligible fraction. In addition, polyphenols-treated cancer cells exhibit activation of both early ROS-dependent and late-ROS-independent genes associated with cell cycle modulation and apoptosis. ROS-dependent genes activated after polyphenols addiction have been identified as early response or biphasic genes [96]. By suppressing H2O2 co-amending cell culture with catalase, only early ROS-dependent apoptosis is abolished, whereas late apoptosis is retarded, as apoptotic cell death still occurred but after a delay of 24 hours. That biphasic-effect on cell apoptosis has been reported by many other studies performed on tea polyphenols [97,98], indicating that polyphenols increase ROS, both sensitive (as H2O2) and insensitive to catalase [99] (as superoxide anions) [100], or, alternatively, they may activate an unrelated ROS-independent apoptotic pathway.
Furthermore, several studies have noticed greater depletions of intracellular GSH in cancer than in normal cells upon their exposures to polyphenols, including grape seed and tea polyphenols extract [101]; in turn, by blocking the recycling of intracellular GSH with an irreversible inhibitor of glutathione reductase, antiproliferative effects of pholyphenols are greatly potentiated [102]. On the contrary, by adding N-acetyl-cysteine – a precursor in the synthesis of glutathione – generation of intracellular ROS was strongly lessened upon exposure of cancer cells to GSE or teaderived polyphenols [103–105]. Flavonoids-induced reduction in GSH availability is however significantly dependent on the dose, given that very low, non-toxic concentrations of quercetin enhance the synthesis of GSH in monkey cancer kidney cells through up regulation of γ-glutamylcysteine synthetase, whereas exposure of cells to high concentrations of GSE or grape/tea polyphenols led to elevated levels of ROS, which quickly depleted GSH stores and thereby increase cellular susceptibility to oxidative free radical attack, resulting in cell death by either apoptosis and/ or necrosis [106,107].
These results may contribute to explain the aforementioned paradoxical behaviour of GSE, highlighting how the pro-oxidant or anti-oxidant effect is context-dependent, as it is shaped by the overall architecture of the redox balance. It should be outlined that such effects have been recorded only for high doses of bioactive compounds, given that a very low GSE concentration (in the range of 1-10 nm) prevents ROS-induced oxidative cell damage, restores intracellular glutathione content, and ameliorates mitochondria-mediated and death receptor-mediated apoptosis in both normal and cancer liver cells [108].
Those effects have been recorded in several cancer lines amended with GSE by ours and other groups [64,103,109–111], and noticed also when using single polyphenolic molecules [112–114]. It is worth noting that the pro-oxidant effect is a very early event (occurring after 5-30 minutes after GSE supplementation), and it happens well before the subsequent onset of apoptosis and cell cycle inhibition. Pre-treatment with N-acetyl-cysteine (NAC) or other ROS-scavenging molecules abolishes almost completely GSE-dependent anticancer effects, and such results indicate that oxidative stress represents a meaningful initiating step. Therefore, GSE-induced oxidative stress in cancer cells should be considered the key-event, preceding the complex molecular cascade leading to GSE-dependent cancer inhibition.
Ros, mitochondrial potential and calcium
GSE, as well as many grape polyphenols and phenolic acids, have been shown to induce significant inhibition of cell proliferation and to enhance apoptosis in several cancer cell lines. Those effects occur at both low and high GSE concentration, the necrotic processes becoming more evident for the highest GSE doses. Such effects have been recently demonstrated to be dependent on ROS formation, occurring early after GSE administration in lung, bladder and colon cancer cells [103,115]. Concomitantly to ROS enhanced formation, the mitochondrial membrane potential was significantly reduced, dose and time-dependently in GSE treated cancer cells [116]. Similar findings have been also reported by adding tea polyphenols to a wide array of cancer cell lines [117]. Those effects were long-lasting, as the decrease in mitochondrial potential still remains after 3-6 hours [118,119]. The mitochondrial transmembrane potential is often used as an indicator of cellular viability and metabolic activity, and its disruption has been involved in a variety of apoptotic phenomena [120].
Moreover, mitochondria have also been implicated in ROS generation during apoptosis. Indeed, reduced mitochondrial membrane potential has recently been shown to lead to increased generation of ROS and apoptosis [121]. Furthermore, mitochondria are central players in cellular Ca2+ signalling given that they contribute in shaping and buffering cellular Ca2+signals [122,123]. It is widely recognised that Ca2+ displays growth inhibiting and differentiation-promoting activities in a variety of normal and malignant epithelial cells. We have reported [103] that intracellular Ca2+ rapidly increased after the addition of GSE to cell cultures. This effect might be due to the mobilisation of intracellular Ca2+ stores, or to the influx of extracellular Ca2+. In order to address these issues, Caco-2 colon cancer cells were incubated in a Ca2+-free medium containing the Ca2+ chelator EGTA, before addiction of GSE obtained from different grape cultivars (Red Globe, Italia and Palieri). Addition of EGTA does not modify intracellular concentration of Ca2+ in Red Globe-treated cells, indicating that modification in intracellular Ca2+ was tightly dependent on extracellular Ca2+ influx in this very case. However, addition of EGTA to the medium supplemented with GSE obtained from Italia and Palieri cultivars, slightly reduced but did not completely inhibit the increase observed in Ca2+ intracellular levels, thus demonstrating that Ca2+ release in these specific cases is largely due to the depletion of intracellular Ca2+ stores. Yet, addition EGTA abolished almost completely GES-induced apoptosis on colon cancer cells as well as mitochondrial depolarisation, thus suggesting the two phenomena are entrenched. Further addition of NAC did not modify significantly those results, suggesting that ROSinduced Ca2+ release is a mandatory step in anticancer effects triggered by GSE. As previously suggested [124], those data outline a crosstalk signalling in between Ca2+and ROS: ROS may regulate the activity of Ca2+-activated channels and, at the same time, increased Ca2+ levels could reinforce ATP synthesis-induced ROS generation. GSE-induced elevation in intracellular calcium levels is also associated to a dramatic downregulation of Calmodulin A (CaM) in breast cancer cells [125]. CaM binds to calcium and hence activates several pathway involved in cancer progression, and increased levels of CaM have been found in cancer cells [126]. However, uncoupled Ca2+ activates the RAF/MEK/ERK pathway and promotes phosphorylation of MAPKp38 and JNK, eventually leading to over-expression of p53 [127].
GSE and Cell-Cyle Modulation
Disruption of the normal regulation of cell-cycle progression and division are important events in the development of cancer. Several proteins are known to regulate the timing of the events in the cell cycle. Major control switches of the cell cycle are the cyclins and the cyclindependent kinases (CDKs). GSE and many of its constituents (chiefly EGCG and resveratrol) [128] have been demonstrated to exert their antiproliferative effects on leukemia [129],ovary [130], lung [131,132] , head and neck [133], prostate [51,134], breast [135,136] and colon cancer, both in vivo and in vitro [137–139]. It is worth noting that, as previously outlined, either native GSE or Tea extract, display a significant greater antiproliferative effect than isolated compounds or synthetic mixtures [140]. GSE treatment resulted in a marked reduction in the expression levels of CDK2, CDK4 and CDK6 [141]. Similarly, a marked reduction in cyclins D1, D2 and E, and an increase expression of negative regulators of the cell cycle (Cdki, such as p21 and p27) were observed after GSE treatment, eventually inducing a dramatic inhibition of cell growth, and a consequent cell cycle arrest in G1, S or G2/M phase [142]. The antiproliferative GSEbased effect involves several molecular targets, including up-regulation of Rb phosphorylation and down-regulation of E2F, through modulation of the EGFR-ERK1/2 pathway [133].
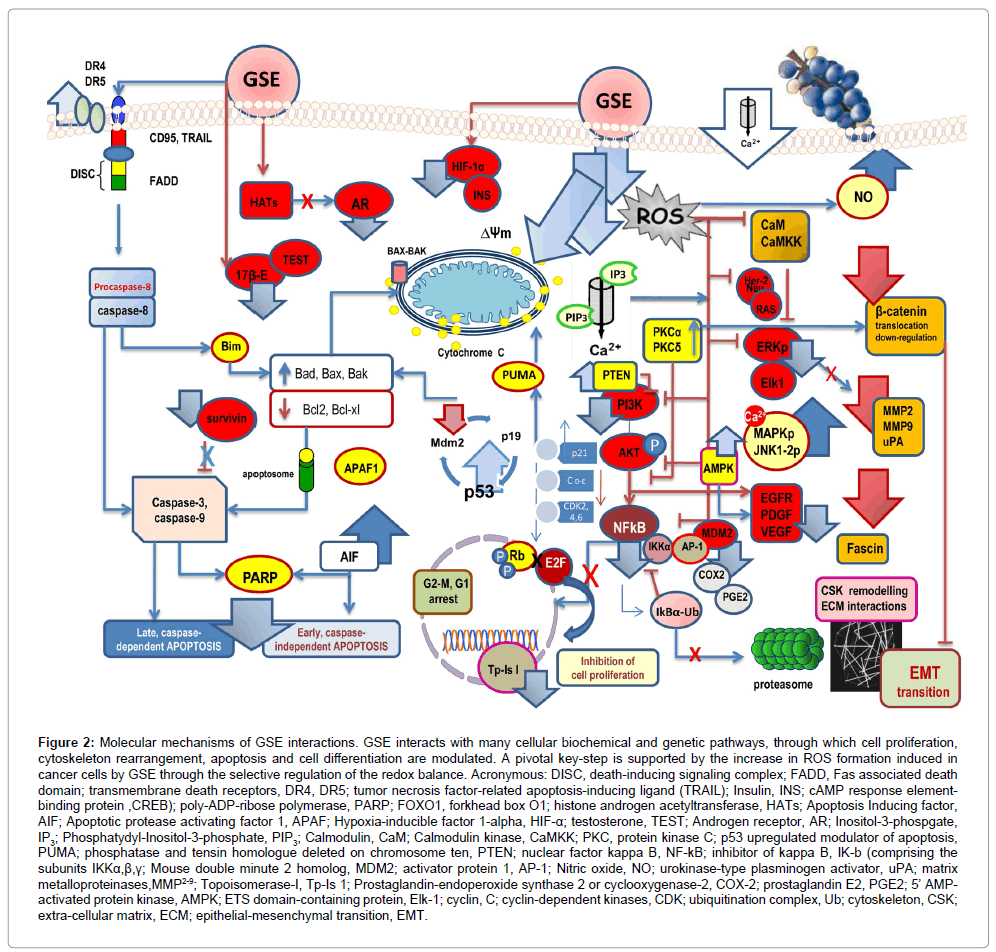
Eventually, those signals converge and activate cyclins, which bind to Cdki to induce cell cycle progression towards S phase. CDKs activity is required to allow cancer progression, and their functions are tightly regulated by Cdki. The increased expressions of Cdki together with decreased expression of cyclins (namely Cyclin-1) [143], and CDKs on GSE-treated cancer cells suggest that GSE might be effective as a chemotherapeutic agent for the treatment of a wide array of tumors. Those effects are likely to involve GSE-mediated inhibition of PI3K/Akt pathway, down-regulation of the epidermal growth factor receptor (EGFR) [144], and interference with NF-kB activity (Figure 2).
Figure 2: Molecular mechanisms of GSE interactions. GSE interacts with many cellular biochemical and genetic pathways, through which cell proliferation, cytoskeleton rearrangement, apoptosis and cell differentiation are modulated. A pivotal key-step is supported by the increase in ROS formation induced in cancer cells by GSE through the selective regulation of the redox balance. Acronymous: DISC, death-inducing signaling complex; FADD, Fas associated death domain; transmembrane death receptors, DR4, DR5; tumor necrosis factor-related apoptosis-inducing ligand (TRAIL); Insulin, INS; cAMP response elementbinding protein ,CREB); poly-ADP-ribose polymerase, PARP; FOXO1, forkhead box O1; histone androgen acetyltransferase, HATs; Apoptosis Inducing factor, AIF; Apoptotic protease activating factor 1, APAF; Hypoxia-inducible factor 1-alpha, HIF-α; testosterone, TEST; Androgen receptor, AR; Inositol-3-phospgate, IP3; Phosphatydyl-Inositol-3-phosphate, PIP3; Calmodulin, CaM; Calmodulin kinase, CaMKK; PKC, protein kinase C; p53 upregulated modulator of apoptosis, PUMA; phosphatase and tensin homologue deleted on chromosome ten, PTEN; nuclear factor kappa B, NF-kB; inhibitor of kappa B, IK-b (comprising the subunits IKKα,β,γ; Mouse double minute 2 homolog, MDM2; activator protein 1, AP-1; Nitric oxide, NO; urokinase-type plasminogen activator, uPA; matrix metalloproteinases,MMP2-9; Topoisomerase-I, Tp-Is 1; Prostaglandin-endoperoxide synthase 2 or cyclooxygenase-2, COX-2; prostaglandin E2, PGE2; 5’ AMPactivated protein kinase, AMPK; ETS domain-containing protein, Elk-1; cyclin, C; cyclin-dependent kinases, CDK; ubiquitination complex, Ub; cytoskeleton, CSK; extra-cellular matrix, ECM; epithelial-mesenchymal transition, EMT.
Pro-Apoptotic Effects of GSE
GSE and MAPK kinases
The extensive investigations with the GSE have identified various molecular targets involved in GSE-mediated cancer cell apoptosis.
The PI3K/Akt pathway plays a pivotal role in mammalian cell survival signaling and has been shown to be activated in various cancers [145]. Indeed, phosphorylated PI3K and Akt are thought to be key factors in modulating down-stream kinases activation and NF-kB-dependent pathways. It is worth of noting that grape and tea polyphenols [146], as well as GSE, have been shown to decrease the PI3K levels and Akt phosphorylation, even enhancing proteasome degradation of Akt in several cancer cell lines [147]. Down-regulation of the phosphorylated form of PI3K is a key event in Akt regulation: Akt binds to phosphatidylinositol- 3-phosphate (PIP3), and PI3K induces its phosphorylation at the carboxy-terminal of Ser473 residue. PI3K is negatively regulated by the phosphorylated form of phosphatase and tensin homologue deleted on chromosome ten (PTEN), a lipid phosphatase that catalyzes the dephosphorylation of PIP3 and thus inhibit PI3K/Akt phosphorylation [148]. Absence of PTEN strongly correlates with activation of PI3K/Akt in tumour cell lines [149], whereas GSE significantly decreased PTEN phosphorylation, and thereby increased its negative regulation on the PI3-K pathway [150]. Phosphorylated Akt may in turn activate survival pathways by directly phosphorylating specific targets. Indeed, Akt negatively regulates factors that promote the expression of death genes (Bad) [151] and positively regulates antiapoptotic factors (Bcl-2, CREB) [152,153] and pro-survival genes (FHKR, NFkB) [154,155]. GSE significantly inhibited Akt-dependent FKHR phosphorylation in Caco-2 cells, thus leading to FHKR proteins residing predominantly in the nucleus where they are able to promote transcription of pro-apoptotic target genes such as Fas-L and Bim through specific DNA elements in their promoters. In addition, GSE suppresses Akt-related effects on CREB, NFkB [135], BAD and Bcl-2, thus promoting an overall pro-apoptotic effect on cancer cells.
MAPKs signaling pathway is an important upstream regulator of transcriptional factor activities and their signaling affects a wide variety of extracellular stimuli into intracellular events and thus control the activities of downstream transcription factors implicated in cancer development and progression [156]. GSE has been reported by many studies to enhance the activation of JNK and p38MAPK, through a pathway requiring intracellular calcium increase [103,157]. In turn, p38MAPK enhances apoptosis through Bcl-2 inactivation, caspase increase and mitochondria depolarization [158]. That effect has been related to ROS [159] and intracellular calcium increase [103], and it is generally thought to participate in enhancing the overall GSE-induced apoptotic action on cancer cells. Yet, opposite findings have been recorded in normal cells [160]. Moreover, GSE and several different polyphenols from both grape and tea have been showed to exert contradictory effects on ERK1/2 activation: meanwhile some studies reported epigallocatechin-3-gallate phosphorylation of ERK1/2 [161], we and others have observed a selective inhibition of ERK phosphorylation in colon and prostate cancer cells treated with GSE [103,147,162,163], or even EGCG [164,165]. Indeed, both down- and up-regulation of ERK activation in cancer cells have been reported occurring after treatment with GSE or isolated polyphenols [115]. Those contradictory results may be ascribed to differences in the cell culture, to dose-dependent dual effects, or to the prevalence of a specific single bioactive component, given that opposite effects on ERK activation have been documented by using different single bioflavonoids [166]. Therefore, data provided by experimental models need to be interpreted according to a systemic approach, i.e. by taking into consideration the dynamic interplay of several other observables [167].
In some way GSE and many dietary pholyphenols seem also to modulate the complex array of PKC iso-enzymes, leading to increased PKCα activation [168]. GSE may activate PKC, namely the PKCα and PCKδ isoforms, probably by increasing intracellular Calcium [169], and promoting PCKδ translocation into the nucleus, where PKC act as proapoptotic factor [170]. PKCα, together with PCKδ, could participate in inhibiting Akt phosphorylation and in triggering the extrinsic apoptotic cascade, especially in prostate cancer cells [171]. However, the interplay in between GSE and PKC dynamics is very poorly understood and deserves further investigation.
Several studies have indicated that elevated levels of inflammation modulators are functionally related to tumor promotion. Prostaglandins are produced in abundance by the metabolic conversion of arachidonic acid by COX-2, which has been known to be upregulated in a number of malignancies. Four transcription factors including nuclear factor kappa B (NF-kB), CCAAT/enhancer-binding protein (C/EBP), activator protein 1 (AP-1) and CRE-binding protein (CREB) have been identified to bind to the cis-acting elements required to promote COX-2 expression [172]. Among the aforementioned factors, NF-kB and AP1 play a relevant role in cancer development and progression [173]. The NF-kB proteins can be activated by a wide variety of stimuli that relieve NF-kB from the inhibition exerted by IkBα. NF-kB is indeed constrained in the cytosol by binding to IkBα. NF-kB activation requires necessarily that this association be disrupted. Almost all activators of NF-kB do so by phosphorylating IkBα when bound to NF-kB–Ikα kinases resulting in accelerated degradation NF-kB and nuclear translocation of free NF-kB [174]. In the nucleus, NFkB targets different gene promoters, enhancing pro-survival pathways and even COX-2 genes expression. In vitro treatment of human epidermoid carcinoma A431 cells with GSE down-regulates the constitutive expression or basal level of NF-κB/p65 and IKKα and simultaneously inhibits the degradation of IκBα protein [175]. Indeed, many polyphenols as well as GSE have been proven to down-regulate NF-kB [136,178–180], and COX-2 expression [179,180]. As for EGCG extracted from tea [181], NFkB down-regulation by GSE may also involve inhibition of Her-2/neu receptor tyrosine phosphorylation, an oncogene member of the EGFR family thought to play a relevant role during cancer development. To our best knowledge, among dietary flavonoids, only EGCG [182], Flavones [183,184] and mangiferin [185] (an apple procyanidin), share with GSE that meaningful, inhibitory property on NF-kB activation. Eventually, GSE has been reported to down-regulate the activator protein-1 (AP-1) levels in cancer cells [186], likely through different, synergistic biochemical pathways, as it was demonstrated by using isolated polyphenols [187]. AP-1 is very often portrayed as a general, nuclear decision maker that determines the final fate of the cell upon stimulation by extracellular signals, and its down-regulation has been claimed to participate in inhibiting antiapoptotic and pro-survival pathways [188].
Additionally GSE and tea polyphenols have been demonstrated to modulate androgen [189] as well as estrogen signalling [190,191], involving a plethora of growth factor, as EFG/EGFR [192], PDGF [193], VEGF [194] and IGFBP-3 [195]. Overall, these effects may converge towards the aforementioned pathways, enhancing the anticancer activity displayed by GSE on cancer cells.
Extrinsic and intrinsic apoptotic pathway
The process of apoptosis is highly complex, and involves a cascade of molecular events, distributed along two main pathways: the extrinsic and the intrinsic or mitochondrial-derived pathway [196]. Both of them interact in some way and eventually converge into the same executioner pathway leading to activation of caspase-3 and PARP [197]. The extrinsic pathway encompasses interactions in between transmembrane death receptors, including DR4 and DR5, two members of the tumor necrosis factor (TNF) receptor gene super family. Cooperative participation of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and the Fas associated death domain (FADD), leads to the formation of the death-inducing signaling complex (DISC), which triggers the autocatalytic cleavage/activation of procaspase-8. Activation of caspase-8 ends-up eventually in activation of terminal caspases (caspase-3) and PARP. On the other hand, intrinsic pathway is activated by several different stimuli, leading to a dramatic decrease in transmembrane mitochondrial potential and consequently release of cytochrome c and pro-apoptotic effectors across the mitochondrial membrane. Further events largely depend on the balance between pro-apoptotic or anti-apoptotic proteins released from mitochondria. Anti-apoptotic effectors include Bcl-2, Bcl-xl; proapoptotic proteins are represented by Bik, Bim, Bak, Bad and Puma. Activation and stabilization of p53, reduced levels of MDM2 and PI3K and phosphorylated Akt, may synergistically shift the equilibrium towards proapoptotic proteins, eventually enhancing the system in moving to the next apoptotic steps. Increased levels of p53, in turn, may lead to augmented levels of Puma, Bak and p21, which translocate into mitochondria reinforcing membrane depolarization and further cytochrome c release and ROS formation [198].
Compelling data have been reported evidencing GSE and many of its bioflavonoids induce apoptosis in cancer cells through both the intrinsic as well as the extrinsic pathway by down-regulating anti-apoptotic proteins and up-regulating several pro-apoptotic factors, eventually leading to activation of caspases 9 and 3 [116,132,137,139,199–201]. Those effects are highly dependent on inhibition of the PI3K/Akt/survivin pathway [202], and on the p38MAPK/JNK/ERK modulation [203], and have been recently confirmed by in vivo studies [204,205]. Moreover, that pro-apoptotic effect is highly specific, as normal cells are generally insensitive to GSE and other dietary polyphenols [137,205,206]. It is worth of noting that such effects occur irrespective of the p53 status of the cells. GSE was originally reported to induce apoptosis in a greater extent in p53-expressing cancer cells (through up-regulation of p53, Bak, p21, and Puma), than in p53-deficient samples [207]. Indeed, some GSE components specifically recognize p53 as a target and lead to p53 activation by binding and interacting to integrin ανβ3 [208]. Furthermore, a selective enhancing effect on p53 has been attributed to both ellagic acid [209] and EGCG [210], which seems to mandatory require p53 to exert its anti-apoptotic effects on cancer cells [211]. However, several studies found that the cytotoxic effect exhibited by the overall extract from grape seeds is actually independent of p53 status of the cancer cell lines [57,133,212]. That observation is highly significant, given the fact that one of the most common genetic defects found in cancers involves deletion/mutation of the TP53 gene, which encodes for the p53 protein [213].
The caspase-dependent pathway might not be the only apoptotic mechanism triggered by GSE (at least in colon cancer cells), bearing in mind that a slightly rise in cleaved PARP may be recorded before an increase in caspase activity could be observed. Indeed, we have shown that apoptosis inducing factor (AIF), known to induce apoptosis via a caspase-independent mechanism, increases early in GSE-treated samples and anticipate caspase-dependent apoptosis [137]. Those results have been further confirmed [109]. Furthermore, both caspase-dependent and caspase-independent apoptosis has been documented in prostate cancer cells after GSE treatment. Even in this case, addition of the ROS-inhibitor NAC prevents almost completely the grape-induced programmed cell death [214]. Involvement of AIF-mediated apoptosis in EGCG-treated cancer cells has also been documented [215,216]. Thus, GSE-induced apoptosis in several cancer cell lines can be considered a biphasic process, obtained through both caspase-dependent and caspase-independent pathways.
Cytoskeleton, ECM-Interactions and EMT-Transition
GSE anticancer actions are not restricted to cell growth inhibition and promotion of apoptosis. Some studies have linked the anti-cancer properties of dietary and tea polyphenols to the induced changes in cytoskeleton architecture and matrix metalloproteinases (MMPs) expression. Indeed, tea flavonoids down-regulate F-actin and 67 kDa-laminin receptor, thus inhibiting the myosin II regulatory light chain [217]. Those effects involve also polyphenols-binding to α2β1-integrin, followed by reorganization of the cytoskeleton, phosphorylation of focal adhesion kinases, and MMPs down-regulation [218].
Similarly, some preliminary reports suggest that cytoskeleton could be a target for GSE activity. Very interesting data have showed that GSE interact with some cytoskeletal proteins, favouring the cytosolic relocalization of β-catenin and down-regulating fascin expression [135]. Fascin is a highly conserved actin-bundling protein that localizes to microspikes and filopodia, participating in motility control [219]. Fascin has been found over-expressed in large numbers of metastatic cancers [229]. Fascin expression is significantly down-regulated by adding GSE to breast cancer cells culture, thus hindering the motility capability of treated cells, as evidenced by the migration assay [135]. Indeed, GSE-treated cancer cells exhibited less motility and invasiveness, a meaningful effect that should be ascribed, at least in part, to the inhibited activity of different MMPs. Conversely, we have observed [135] that GSE greatly inhibited MMP-2 and MMP-9 expression, as well as urokinase-type plasminogen activator (uPA), a key factor which mediates cellular invasion both directly by degrading members of the matrix proteins [221] and indirectly by modulating MMPs activation. A similar pattern has been observed by treating cancer cells with resveratrol [222], resveratrol analogues [223], or other dietary proto-anthocyanidins [224] and polyphenols [225–227]. It is interesting that effects on metalloproteinases, at least when referred to EGCG-treated cancer cells, have been deemed as a consequence of increased release of super oxygen radicals [228]. Additionally, it has been reported that GSE enhances the levels of epithelial (E-cadherin, cytokeratins and desmoglein-2) and reduces the levels of mesenchymal (vimentin, fibronectin, N-cadherin and Slug) biomarkers [144]. Overall those results suggest that GSE may efficiently counteract the epithelial-mesenchymal transition as well as the invasivity of cancer cells, by remodelling the cell-matrix interactions and stabilizing collagen architecture [229,230]. Those findings shed light on the mechanisms supporting the observed anti-metastatic property of GSE. Indeed, preliminary experimental investigations on animals showed that GSE significantly inhibits lung [136] and bone [231] metastasis from mouse breast cancers. Such preliminary results disclose a very new perspective in understanding the anticancer effects displayed by GSE.
Conclusion
Since ancient times, in various cultures and religions, there has been a strong belief that alcohol offers important health benefits. In recent years, the idea that regular, moderate alcohol consumption protects against cardiovascular disease and degenerative diseases has gained momentum. A large number of studies have shown a significant inverse relationship between wine and/or grape consumption and mortality from all causes [232]. Specifically, a moderate wine consumption as well as regular intake of grape fruits seem to ensure an overall benefit in reducing the risk of dying from heart disease or cancer by approximately 40-60% [233,234]. During the last two decades, those data received a convincingly confirmation by a huge body of experimental investigations carried out in vitro as well in vivo models [235,236], demonstrating how GSE can hamper carcinogenesis and even counteract cancer development and progression, by inducing apoptosis and cell cycle arrest.
Is rather paradoxical that GSE promotes such effects by first enhancing intracellular ROS. ROS are thought to play a relevant role at the very beginning of the carcinogenic process, indeed. However, they behave really akin a double-edged sword, given that when their intracellular levels overwhelm the cellular antioxidant capacity, ROS increase ends up being detrimental to cells survival [237]. By taking into consideration how important ROS-induced apoptosis could be in improving current therapeutic anticancer strategies without adversely affecting normal cells [238], it can be concluded that GSE supplementation promises to be a reliable, new pharmacological opportunity that deserves much more thoughtfulness in both experimental and clinical studies. Indeed, GSE have shown that it is well tolerated and is considered safe as dietary supplement for human consumption, even at the highest doses and for long-lasting period of administration [239,240].
Both epidemiological and experimental studies currently support the beneficial effects of dietary pholyphenols and namely, of GSE. Several bioactive compounds have been identified and their activity has been documented, both in vitro and in vivo. However, the key question here is whether a purified component (whatsoever its effectiveness should be) has the same health benefit of the mixture from which it has been extracted. Indeed, it has long be recognized that GSE displays synergistic effects and additive interactions that potentiate the activity of individual components, thus suggesting that these compounds will exert their bioactivities only when harvested or delivered as natural mixtures from plant cell donors [241,242]. Likewise, although most experimental data have demonstrated the relevant anticancer role sustained by EGCG as the prevalent green tea constituent, the overall biological activity of green tea is thought to require the cooperative action of several components, rather than a single molecule [243,244]. Dietary phytochemicals are generally embedded into complex mixtures that often act in a synergistic fashion [245,246]: the isolated pure compound either loses its bioactivity, becomes unstable, or may not behave the same way as the compound in whole foods, as it has been demonstrated for a lot of phytochemicals, including the well-known anti-oxidant vitamin C [247,248]. In addition, diversity in molecular size, polarity, and solubility, may affect the bioavailability and distribution of each phytochemicals in different macromolecules, subcellular organelles, cells, and even tissues. Those considerations may explain the contradictory, even paradoxical results obtained in chemopreventive trials by using purified, single compounds [249,250]: no single molecule can replace the combination of natural phytochemicals in fruits, vegetables or overall grape seed extract in achieving the observed health benefits.
Thus, it is now “widely believed that the actions of the dietary supplements alone do not explain the observed health benefits of diets rich in fruits, vegetables, and whole grain, because, taken alone, individual molecules studied in clinical trials do not appear to have consistent preventive effects” [251].
Compelling data strongly suggest that grapes and grape-based products exert significant anticancer effects, as demonstrated by studies performed on cell culture and animal models. Grape polyphenols enhance ROS levels in cancer cells, leading then to a wide array of molecular and genetic changes, including phosphorylation of MAP-kinases, inhibition of PI3K-Akt and NF-kB pathways, down-regulation of cyclins and CDKs, and activation of both extrinsic and intrinsic apoptotic pathways. By that way, GSE selectively hinders cell proliferation and strongly enhances apoptosis. Despite the many challenges for dietary natural products caused by lack of standardization, composition variability and the limited reporting of adverse effects [252], such ‘mixtures’ have a great relevance as source of potential new pharmacological molecules and may represent an important opportunity for clinical research that should not be neglected. Considering the limited therapeutic options still available against several types of cancer, results herein reported indicate GSE could be thought as a new valuable treatment. Yet, clinical studies are urgently warranted in order to support this attractive hypothesis.
References
- Key TJ, Allen NE, Spencer EA, Travis RC (2002) The effect of diet on risk of cancer. Lancet 360: 861-868.
- Glade MJ (1999) Food, nutrition, and the prevention of cancer: a global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition 15: 523-526.
- Vidavalur R, Otani H, Singal PK, Maulik N (2006) Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol 11: 217-225.
- Pezzuto JM (2008) Grapes and human health: a perspective. J Agric Food Chem 56: 6777-6784.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, et al. (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155: 381-386.
- Yang CS, Landau JM, Huang MT, Newmark HL (2001) Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 21: 381-406.
- German JB (1997) Nutritional studies of flavonoids in wine: Flavonoids in Health and Disease: Rice-Evans CA, Packer L (Eds), Marcel Dekker, New York.
- Ruano-Ravina A, Figueiras A, Barros-Dios JM (2004) Type of wine and risk of lung cancer: a case-control study in Spain. Thorax 59: 981-985.
- Chao C (2007) Associations between beer, wine, and liquor consumption and lung cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 16: 2436-2447.
- De Stefani E, Correa P, Deneo-Pellegrini H, Boffetta P, Gutiérrez LP, et al. (2002) Alcohol intake and risk of adenocarcinoma of the lung. A case-control study in Uruguay. Lung Cancer 38: 9-14.
- Yang CS (1997) Inhibition of carcinogenesis by tea. Nature 389: 134-135.
- Sun CL, Yuan JM, Koh WP, Yu MC (2006) Green tea, black tea and breast cancer risk: a meta-analysis of epidemiological studies. Carcinogenesis 27: 1310-1315.
- Suzuki Y, Tsubono Y, Nakaya N, Suzuki Y, Koizumi Y, et al. (2004) Green tea and the risk of breast cancer: pooled analysis of two prospective studies in Japan. Br J Cancer 90: 1361-1363.
- Kuroda Y, Hara Y (1999) Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res 436: 69-97.
- Crupi P, Coletta A, Anna Milella R, Perniola R, Gasparro M, et al. (2012) HPLC-DAD-ESI-MS analysis of flavonoid compounds in 5 seedless table grapes grown in Apulian Region. J Food Sci 77: C174-181.
- Morré DM, Morré DJ (2006) Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett 238: 202-209.
- Frederiksen H, Mortensen A, Schrøder M, Frandsen H, Bysted A, et al. (2007) Effects of red grape skin and seed extract supplementation on atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Mol Nutr Food Res 51: 564-571.
- Zern TL, Wood RJ, Greene C, West KL, Liu Y, et al. (2005) Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr 135: 1911-1917.
- Alonso Borbalán AM, Zorro L, Guillén DA, Barroso CG (2003). Study of the polyphenol content of red and white grape varieties by liquid chromatographymass spectrometry and its relationship to antioxidant power. J Chromatogr A, 1012: 31-38.
- Castillo-Muñoz N, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I (2007) Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J Agric Food Chem 55: 992-1002.
- Cavaliere C, Foglia P, Marini F, Samperi R, Antonacci D.et al. (2010) The interactive effects of irrigation, nitrogen fertilisation rate, delayed harvest and storage on the polyphenol content in red grape (Vitis vinifera) berries: A factorial experimental design. Food Chem 122: 1176-1184.
- Santos-Buelga C, Francia-Aricha EM, Escribano-Bailòn MT (1995) Comparative flavan-3-ol composition of seeds from different grape varieties. Food Chem 53: 197-201.
- Cantos E, Espín JC, Tomás-Barberán FA (2002) Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J Agric Food Chem 50: 5691-5696.
- Kennedy JA, Matthews MA, Waterhouse AL (2002) Effect of maturity and vine water status on grape skin and wine flavonoids. Am J Enol Vitic 53: 268-274.
- Kennedy JA, Matthews MA, Waterhouse AL (2000) Changes in grape seed polyphenols during fruit ripening. Phytochemistry 55: 77-85.
- Esteban MA; Villanueva MJ; Lissarrague JR (2001) Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L) grape berries during ripening. J Sci Food Agric 81: 409-420.
- Perez-Magariño S, González-San José MA (2006) Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem 96: 197-208.
- Choné X, Lavigne-Cruège V, Tominaga T, Van Leeuwen C, Castagnède, et al. (2006) Effect of vine nitrogen status on grape aromatic potential: flavour precursors (s-cysteineconjugates),glutathione and phenolic content in Vitis vinifera L. cv. Sauvignon blanc grape juice. J int Sci Vigne Vin 40: 1-6.
- Pekic B, Kovac V, Alonso E, Revilla E (1998) Study of the extraction of proanthocyanidins from grape seeds. Food Chem 61: 201-206.
- Mandic AI, Dilas SM, Cetkovic GS, Canadanovic-Brunet JM, Tumbas VT (2008) Polyphenolic Composition and Antioxidant Activities of Grape Seed Extract. Int J Food Properties 11: 713-726.
- Yilmaz Y, Toledo RT (2006) Oxygen radical absorbance capacities of grape/wine industry by products and effect of solvent type on extraction of grape seed Polyphenols. J Food Comp Anal 19: 41-48
- Weidner S, Karamac M, Amarowicz R, Szypulska E, Golgowska A (2007) Changes in composition of phenolic compounds and antioxidant properties of Vitis amurensis seeds germinated under osmotic stress. Acta Physiol Plant 29: 283-290.
- Bartosz G (1997) Oxidative stress in plants. Acta Physiol Plant 19: 47-64.
- Farrant JM, Bailly C, Leymarie J, Hamman B, Côme D, et al. (2004) Wheat seedlings as a model to understand desiccation tolerance and sensitivity. Physiol Plant 120: 563-574.
- Cho YJ, Kim JE, Chun HS, Kim CT, Kim SS, et al. (2003) Contents of resveratrol in different parts of grapes. Korean J Food Sci and Technol 35: 306-308.
- Butkhup L, Chowtivannakul S, Gaensakoo R, Prathepha P, Samappito S (2010) Study of the phenolic composition of Shiraz red grape cultivar (Vitis vinifera L.) cultivated in North-eastern Thailand and its antioxidant and antimicrobial activity. S Afr J EnoL Vitic.31: 89-98.
- Hughes RJ, Croley TR, Metcalfe CD, March RE (2001) A tandem mass spectrometric study of selected characteristic flavonoids. Int J Mass Spectrom 210: 371-385.
- Hollman PC, Katan MB (1999) Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol 37: 937-942.
- Shi J, Yu J, Pohorly JE, Kakuda Y (2003) Polyphenolics in grape seeds-biochemistry and functionality. J Med Food 6: 291-299.
- Iacopini P, Baldi M, Storchi P, Sebastiani L (2008) Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. Journal of Food Compost Anal 21: 589-598.
- Bors W, Saran M (1987) Radical scavenging by flavonoid antioxidants. Free Radic Res Commun 2: 289-294.
- Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR (1988) Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol 40: 787-792.
- Landolfi R, Mower RL, Steiner M (1984) Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure-activity relations. Biochem Pharmacol 33: 1525-1530.
- Capasso R, Evidente A, Schivo L, Orru G, Marcialis MA, et al. (1995) Antibacterial polyphenols from olive oil mill waste waters. J Appl Bacteriol 79: 393-398.
- Rice-Evans C (2001) Flavonoid antioxidants. Curr Med Chem 8: 797-807.
- Arnous A, Makris DP, Kefalas P (2001) Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem 49: 5736-5742.
- Landbo AK; Meyer AS (2001) Ascorbic acid improves the antioxidant activity of European grape juices by improving the juices’ ability to inhibit lipid peroxidation of human LDL in vitro. Int J Food Sci Technol 36: 727-735.
- Vattem DA, Shetty K (2005) Biological functionality of ellagic acid: a review. J Food Biochem 29: 234-266.
- Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, et al. (2006) Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 27: 1445-1453.
- Pan MH, Chiou YS, Wang YJ, Ho CT, Lin JK (2011) Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct 2: 101-110.
- Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, et al. (2007) Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis 28:1478-1484.
- ElAttar TM, Virji AS (1999) Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs 10: 187-193.
- Bakkalbas E, Yemis O, Aslanova D, Artik N (2005) Major flavan-3-ol composition and antioxidant activity of seeds from different grape cultivars grown in Turkey. Eur Food Res Technol 221: 792–797.
- Guendez R, Kallithraka S, Makris DP, Kefalas P (2005) Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chemistry 89: 1-9
- Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, et al. (2000) The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr 72: 1150-1155.
- Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, et al. (2013) Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem Toxicol 61: 60-68.
- Dinicola S, Cucina A, Pasqualato A, D’Anselmi F, Proietti S, et al. (2012) Antiproliferative and Apoptotic Effects Triggered by Grape Seed Extract (GSE) versus Epigallocatechin and Procyanidins on Colon Cancer Cell Lines. Int J Mol Sci 13: 651-664.
- Zhao J, Wang J, Chen Y, Agarwal R (1999) Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis 20: 1737-1745.
- Singletary KW, Meline B (2001) Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer 39: 252-258.
- Kim H, Hall P, Smith M, Kirk M, Prasain JK, et al. (2004) Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr 134: 3445S-3452S.
- Chang J (2000) Medicinal herbs: drugs or dietary supplements? Biochem Pharmacol 59: 211-219.
- Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, et al. (1999) The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem 196: 99-108.
- Mittal A, Elmets CA, Katiyar SK (2003) Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis 24: 1379-1388.
- Shrotriya S, Deep G, Gu M, Kaur M, Jain AK, et al. (2012) Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis 33: 848-858.
- Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, et al. (2000) Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology 148: 187-197.
- Yilmaz Y, Toledo RT (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem 52: 255-260.
- Stagos D, Kazantzoglou G, Theofanidou D, Kakalopoulou G, Magiatis P, et al. (2006). Activity of grape extracts from Greek varieties of Vitis vinifera against mutagenicity induced by bleomycin and hydrogen peroxide in Salmonella typhimurium strain TA102. Mutat Res 609: 165-175.
- Jayaprakasha GK, Singh RP, Sakariah KK (2001) Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry 73: 285-290.
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, et al. (1997) Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol 95: 179-189.
- Enginar H, Cemek M, Karaca T, Unak P (2007) Effect of grape seed extract on lipid peroxidation, antioxidant activity and peripheral blood lymphocytes in rats exposed to x-radiation. Phytother Res 21: 1029-1035.
- Choi SK, Zhang XH, Seo JS (2012) Suppression of oxidative stress by grape seed supplementation in rats. Nutr Res Pract 6: 3-8.
- Cetin A, Kaynar L, Koçyiğit I, Hacioğlu SK, Saraymen R, et al. (2008) The effect of grape seed extract on radiation-induced oxidative stress in the rat liver. Turk J Gastroenterol 19: 92-98.
- Khan SG, Katiyar SK, Agarwal R, Mukhtar H (1992) Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res 52: 4050-4052.
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, et al. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313-322.
- Chen C, Yu R, Owuor ED, Kong AN (2000) Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res 23: 605-612.
- Kong AN, Owuor E, Yu R, Hebbar V, Chen C, et al. (2001) Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab Rev 33: 255-271.
- Kar P, Laight D, Rooprai HK, Shaw KM, Cummings M (2009) Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: a double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet Med 26: 526-531.
- Vinson JA, Proch J, Bose P (2001) MegaNatural((R)) Gold Grapeseed Extract: In Vitro Antioxidant and In Vivo Human Supplementation Studies. J Med Food 4: 17-26.
- Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, et al. (2006) Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr 84: 252-262.
- Hogan S, Canning C, Sun S, Sun X, Zhou K (2010) Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J Agric Food Chem 58: 11250-11256
- Halliwell B, Gutteridge JMC (1999) Free Radicals in Biology and Medicine (second ed), Oxford University Press, Oxford.
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239-247.
- Matés JM, Sánchez-Jiménez FM (2000) Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol 32: 157-170.
- López-Oliva ME, Agis-Torres A, Goñi I, Muñoz-Martínez E (2010) Grape antioxidant dietary fibre reduced apoptosis and induced a pro-reducing shift in the glutathione redox state of the rat proximal colonic mucosa. Br J Nutr 103: 1110-1117.
- Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141: 312-322.
- Bombardelli E, Morazzoni P, Carini M, Aldini G, Maffei Facino R (1997) Biological activity of procyanidins from Vitis vinifera L. BioFactors 6: 429-431.
- Cutter H, Wu LY, Kim C, Morré DJ, Morré DM (2001) Is the cancer protective effect correlated with growth inhibitions by green tea (-)-epigallocatechin gallate mediated through an antioxidant mechanism? Cancer Lett 162: 149-154.
- Weisburg JH, Weissman DB, Sedaghat T, Babich H (2004) In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin Pharmacol Toxicol 95: 191-200.
- Troyano A, Fernández C, Sancho P, de Blas E, Aller P (2001) Effect of glutathione depletion on antitumor drug toxicity (apoptosis and necrosis) in U-937 human promonocytic cells. The role of intracellular oxidation. J Biol Chem 276: 47107-47115.
- Ignea C, Dorobanţu CM, Mintoff CP, Branza-Nichita N, Ladomery MR, et al. (2013) Modulation of the antioxidant/pro-oxidant balance, cytotoxicity and antiviral actions of grape seed extracts. Food Chem 141: 3967-3976.
- Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, et al. (2005) Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J 19: 807-809.
- Babich H, Schuck AG, Weisburg JH, Zuckerbraun HL (2011) Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J Toxicol 2011: 467305.
- Yang GY, Liao J, Li C, Chung J, Yurkow EJ, et al. (2000) Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 21: 2035-2039.
- Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS (1998) Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19: 611-616.
- Chai PC, Long LH, Halliwell B (2003) Contribution of hydrogen peroxide to the cytotoxicity of green tea and red wines. Biochem Biophys Res Commun 304: 650-654.
- Vittal R, Selvanayagam ZE, Sun Y, Hong J, Liu F, et al. (2004) Gene expression changes induced by green tea polyphenol (-)-epigallocatechin-3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol Cancer Ther 3: 1091-1099.
- Babich H, Zuckerbraun HL, Weinerman SM (2007) In vitro cytotoxicity of (-)-catechin gallate, a minor polyphenol in green tea. Toxicol Lett 171: 171-180.
- Yamamoto T, Lewis J, Wataha J, Dickinson D, Singh B, et al. (2004) Roles of catalase and hydrogen peroxide in green tea polyphenol-induced chemopreventive effects. J Pharmacol Exp Ther 308: 317-323.
- Wenzel U, Schoberl K, Lohner K, Daniel H (2005) Activation of mitochondrial lactate uptake by flavone induces apoptosis in human colon cancer cells. J Cell Physiol 202: 379-390.
- Juan ME, Wenzel U, Daniel H, Planas JM (2008) Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem 56: 4813-4818.
- Babich H, Selevan AR, Ravkin ER (2007) Glutathione as a mediator of the in vitro cytotoxicity of a green tea polyphenol extract. Toxicol Mech Methods 17: 357-369.
- Babich H, Sedletcaia A, Kenigsberg B (2002) In vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: involvement in oxidative stress. Pharmacol Toxicol 91: 245-253.
- Dinicola S, Mariggiò MA, Morabito C, Guarnieri S, Cucina A, et al. (2013) Grape seed extract triggers apoptosis in Caco-2 human colon cancer cells through reactive oxygen species and calcium increase: extracellular signal-regulated kinase involvement. Br J Nutr 110:797-809.
- Ishino A, Mita S, Watanabe S, Sakagami H (1999) Effect of anticancer drugs, metals and antioxidants on cytotoxic activity of epigallocatechin gallate. Anticancer Res 19: 4343-4348.
- Atsumi T, Tonosaki K, Fujisawa S (2006) Induction of early apoptosis and ROS-generation activity in human gingival fibroblasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch Oral Biol 51: 913-921.
- Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ (2002) Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med 32: 386-393.
- Kennedy DO, Matsumoto M, Kojima A, Matsui-Yuasa I (1999) Cellular thiols status and cell death in the effect of green tea polyphenols in ehrlich ascites tumor cells. Chem Biol Interact 122: 59-71.
- Xu Y, Khaoustov VI, Wang H, Yu J, Tabassam F, et al. (2009) Freeze-dried grape powder attenuates mitochondria- and oxidative stress-mediated apoptosis in liver cells. J Agric Food Chem 57: 9324-9331.
- Derry M, Raina K, Agarwal R, Agarwal C (2012) Differential effects of grape seed extract against human colorectal cancer cell lines: The intricate role of death receptors and mitochondria. Cancer Lett .
- Raina K, Tyagi A, Kumar D, Agarwal R, Agarwal C (2013) Role of oxidative stress in cytotoxicity of grape seed extract in human bladder cancer cells. Food Chem Toxicol 61: 187-195.
- Tyagi A, Raina K, Gangar S, Kaur M, Agarwal R, et al. (2013) Differential effect of grape seed extract against human non-small-cell lung cancer cells: the role of reactive oxygen species and apoptosis induction. Nutr Cancer 65 Suppl 1: 44-53.
- Nakazato T, Ito K, Miyakawa Y, Kinjo K, Yamada T, et al. (2005) Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica 90: 317-325.
- Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, et al. (2000) Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun 270: 793-797.
- Sergediene E, Jönsson K, Szymusiak H, Tyrakowska B, Rietjens IM, et al. (1999) Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett 462: 392-396.
- Kaur M, Tyagi A, Singh RP, Sclafani RA, Agarwal R, et al. (2011) Grape seed extract upregulates p21 (Cip1) through redox-mediated activation of ERK1/2 and posttranscriptional regulation leading to cell cycle arrest in colon carcinoma HT29 cells. Mol Carcinog 50: 553-562.
- Hsu CP, Lin YH, Chou CC, Zhou SP, Hsu YC, et al. (2009) Mechanisms of grape seed procyanidin-induced apoptosis in colorectal carcinoma cells. Anticancer Res 29: 283-289.
- Hsuuw YD, Chan WH (2007) Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann N Y Acad Sci 1095: 428-440.
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C (2006) Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res 12: 6194-6202.
- Meeran SM, Katiyar SK (2007) Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp Dermatol 16: 405-415.
- Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, et al. (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184: 1155-1160.
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, et al. (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182: 367-377.
- Eager KR, Roden LD, Dulhunty AF (1997) Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am J Physiol 272: C1908-1918.
- Boitier E, Rea R, Duchen MR (1999) Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol 145: 795-808.
- Feissner RF, Skalska J, Gaum WE, Sheu SS (2009) Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14: 1197-1218.
- Hakimuddin F, Paliyath G, Meckling K (2004) Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat 85: 65-79.
- Veigl ML, Vanaman TC, Sedwick WD (1984) Calcium and calmodulin in cell growth and transformation. Biochim Biophys Acta 738: 21-48.
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, et al. (2005) Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell 16: 4437-4453.
- Cardile V, Lombardo L, Spatafora C, Tringali C (2005) Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorg Chem 33: 22-33.
- Kang JH, Park YH, Choi SW, Yang EK, Lee WJ (2003) Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp Mol Med 35: 467-474.
- Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, et al. (2005) Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis 26: 1978-1987.
- Akhtar S, Meeran SM, Katiyar N, Katiyar SK (2009) Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res 15: 821-831.
- Singh T, Sharma SD, Katiyar SK (2011) Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS One 6: e27444.
- Prasad R, Katiyar SK (2012) Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS One 7: e46404.
- Raina K, Singh RP, Agarwal R, Agarwal C (2007) Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res 67: 5976-5982.
- Dinicola S, Pasqualato A, Cucina A, Coluccia P, Ferranti F, et al. (2013) Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur J Nutr .
- Mantena SK, Baliga MS, Katiyar SK (2006) Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 27: 1682-1691.
- Dinicola S, Cucina A, Pasqualato A, Proietti S, D’Anselmi F, et al. (2010) Apoptosis-inducing factor and caspase-dependent apoptotic pathways triggered by different grape seed extracts on human colon cancer cell line Caco-2. Br J Nutr 104: 824-832.
- Marel AK, Lizard G, Izard JC, Latruffe N, Delmas D (2008) Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol Nutr Food Res 52: 538-548.
- Kaur M, Mandair R, Agarwal R, Agarwal C (2008) Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr Cancer 60 Suppl 1: 2-11.
- Morré DJ, Morré DM, Sun H, Cooper R, Chang J, et al. (2003) Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX). Pharmacol Toxicol 92: 234-241.
- Meeran SM, Katiyar SK (2008) Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front Biosci 13: 2191-2202.
- Laurent C, Besançon P, Auger C, Rouanet JM, Caporiccio B (2004) Grape seed extract affects proliferation and differentiation of human intestinal Caco-2 cells. J Agric Food Chem 52: 3301-3308.
- Lim YC, Lee SH, Song MH, Yamaguchi K, Yoon JH, et al. (2006) Growth inhibition and apoptosis by (-)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer 42: 3260-3266.
- Sun Q, Prasad R, Rosenthal E, Katiyar SK (2011) Grape seed proanthocyanidins inhibit the invasive potential of head and neck cutaneous squamous cell carcinoma cells by targeting EGFR expression and epithelial-to-mesenchymal transition. BMC Complement Altern Med 11: 134.
- Chang F, Lee JT, Navolonic PM, Steelman LS, Shelton JG, et al. (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17: 590-603
- Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, et al. (2007) A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun 354: 852-857.
- Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, et al. (2007) Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res 67: 8396-8405.
- Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382: 1-11.
- Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL (1998) The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci U S A 95: 15587-15591.
- Engelbrecht AM, Mattheyse M, Ellis B, Loos B, Thomas M, et al. (2007) Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett 258: 144-153.
- Tan Y, Demeter MR, Ruan H, Comb MJ (2000) BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem 275: 25865-25869.
- Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, et al. (1997) Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J 16: 6151-6161.
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, et al. (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275: 10761-10766.
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, et al. (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857-868.
- Dolcet X, Llobet D, Pallares J, Matias-Guiu X (2005) NF-kB in development and progression of human cancer. Virchows Arch 446: 475-482.
- Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9: 537-549.
- Chen KC, Liu WH, Kao PH, Chang LS (2010) Calcium-stimulated mitogen-activated protein kinase activation elicits Bcl-xL downregulation and Bak upregulation in notexin-treated human neuroblastoma SK-N-SH cells. J Cell Physiol 222: 177-186.
- De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, et al. (2006) Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem 281: 21353-21361.
- Chen ZP, Schell JB, Ho CT, Chen KY (1998) Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett 129: 173-179.
- Balasubramanian S, Efimova T, Eckert RL (2002) Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem 277: 1828-1836.
- Siddiqui IA, Adhami VM, Afaq F, Ahmad N, Mukhtar H (2004) Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J Cell Biochem 91: 232-242.
- Briviba K, Pan L, Rechkemmer G (2002) Red wine polyphenols inhibit the growth of colon carcinoma cells and modulate the activation pattern of mitogen-activated protein kinases. J Nutr 132: 2814-2818.
- Tyagi A, Agarwal R, Agarwal C (2003) Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene 22: 1302-1316.
- Eberhardt W, Huwiler A, Beck K-F, Walpen S, Pfeilschifter J (2000) Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol 165: 5788–5797.
- Opare Kennedy D, Kojima A, Hasuma T, Yano Y, Otani S, et al. (2001) Growth inhibitory effect of green tea extract and (-)-epigallocatechin in ehrlich ascites tumor cells involves a cellular thiol-dependent activation of mitogenicactivated protein kinases. Chem Biol Interact 134: 113-133
- Kong AN, Yu R, Hebbar V, Chen C, Owuor E, et al. (2001) Signal transduction events elicited by cancer prevention compounds. Mutat Res 480-481: 231-41.
- Bizzarri M, Palombo A, Cucina A (2013) Theoretical aspects of Systems Biology. Prog Biophys Mol Biol 112: 33-43.
- Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, et al. (2005) Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 26: 1291-1295.
- Sampson SR, Lupowitz Z, Braiman L, Zisapel N (2006) Role of protein kinase Calpha in melatonin signal transduction. Mol Cell Endocrinol 252: 82-87.
- Zhang X-M, Chen J, Xia Y-G, Xu Q (2005) Apoptosis of murine melanoma B16-BL6 cells induced by quercetin targeting mitochondria, inhibiting expression of PKC-alfa and translocating PKC-delta. Cancer Chemother Pharmacol 55: 251-262.
- Gonzalez-Guerrico AM, Kazanietz MG (2005) Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J Biol Chem 280: 38982-38991.
- Kundu JK, Shin YK, Surh YJ (2006) Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol 72: 1506-1515.
- Perkins ND (2007) Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8: 49-62.
- Aggarwal BB (2004) Nuclear factor-kappaB: the enemy within. Cancer Cell 6: 203-208.
- Meeran SM, Katiyar SK (2008) Proanthocyanidins inhibit mitogenic and survival-signaling in vitro and tumor growth in vivo. Front Biosci 13: 887-897.
- Vayalil PK, Mittal A, Katiyar SK (2004) Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NF kappa B. Carcinogenesis 25: 987-995.
- Vayalil PK, Katiyar SK (2004) Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate 59: 33-42.
- Holmes-McNary M, Baldwin AS Jr (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 60: 3477-3483.
- Velmurugan B, Singh RP, Kaul N, Agarwal R, Agarwal C (2010) Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice. Neoplasia 12: 95-102.
- Mutoh M, Takahashi M, Fukuda K, Matsushima-Hibiya Y, Mutoh H, et al. (2000) Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 21: 959-963.
- Pianetti S, Guo S, Kavanagh KT, Sonenshein GE (2002) Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res 62: 652-655.
- Ahmad N, Gupta S, Mukhtar H (2000) Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys 376: 338-346.
- Kalra N, Seth K, Prasad S, Singh M, Pant AB, et al. (2007) Theaflavins induced apoptosis of LNCaP cells is mediated through induction of p53, down-regulation of NF-kappa B and mitogen-activated protein kinases pathways. Life Sci 80: 2137-2146.
- Wenzel U, Kuntz S, Brendel MD, Daniel H (2000) Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res 60: 3823-3831.
- Sarkar A, Sreenivasan Y, Ramesh G, Manna S (2004) beta-D-glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor kappaB but potentiates apoptosis. J Biol Chem 279: 33768-33781.
- Gopalakrishnan A, Tony Kong AN (2008) Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol 46: 1257-1270.
- Chung JY, Huang C, Meng X, Dong Z, Yang CS (1999) Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res 59: 4610-4617
- Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859-868.
- Liao S (2001) The medicinal action of androgens and green tea epigallocatechin gallate. Hong Kong Med J 7: 369-374.
- Kampa M, Theodoropoulou K, Mavromati F, Pelekanou V, Notas G, et al. (2011) Novel oligomeric proanthocyanidin derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer. J Pharmacol Exp Ther 337: 24-32.
- Kao YH, Hiipakka RA, Liao S (2000) Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 141: 980-987.
- Sun Q, Prasad R, Rosenthal E, Katiyar SK (2012) Grape seed proanthocyanidins inhibit the invasiveness of human HNSCC cells by targeting EGFR and reversing the epithelial-to-mesenchymal transition. PLoS One 7: e31093.
- Labrecque L, Lamy S, Chapus A, Mihoubi S, Durocher Y, et al. (2005) Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis 26: 821-826.
- Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, et al. (2007) Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett 247: 115-121.
- Barthomeuf C, Lamy S, Blanchette M, Boivin D, Gingras D, et al. (2006) Inhibition of sphingosine-1-phosphate- and vascular endothelial growth factor-induced endothelial cell chemotaxis by red grape skin polyphenols correlates with a decrease in early platelet-activating factor synthesis. Free Radic Biol Med 40:581-590.
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495-516.
- Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2: 277-288.
- Schuler M, Green DR (2001) Mechanisms of p53-dependent apoptosis. Biochem Soc Trans 29: 684-688.
- Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, et al. (2001) Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun 285: 1102-1106.
- Agarwal C, Singh RP, Agarwal R. (2002) Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 23: 1869-1876.
- Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, et al. (2005) Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol 115: 186-191.
- Tang Y, Zhao DY, Elliott S, Zhao W, Curiel TJ, et al. (2007) Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int J Oncol 31: 705-711.
- Kaur M, Agarwal C, Agarwal R (2009) Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr 139: 1806S-12S.
- Prasad R, Vaid M, Katiyar SK (2012) Grape proanthocyanidin inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway. PLoS One 7: e43064.
- Park HK, Han DW, Park YH, Park JC (2005) Differential biological responses of green tea polyphenol in normal cells vs. cancer cells. Curr Appl Phys 5: 449-452
- Feng R, Ni HM, Wang SY, Tourkova IL, Shurin MR, et al. (2007) Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J Biol Chem 282: 13468-13476.
- Roy AM, Baliga MS, Elmets CA, Katiyar SK (2005) Grape seed proanthocyanidins induce apoptosis through p53, Bax, and caspase 3 pathways. Neoplasia 7: 24-36.
- Hsieh TC, Wong C, John Bennett D, Wu JM (2011) Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: an in silico and biochemical approach targeting integrin αvβ3. Int J Cancer 129: 2732-2743.
- Mertens-Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218: 141-151.
- Manna S, Banerjee S, Mukherjee S, Das S, Panda CK (2006) Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: mediated by p53 pathway and inhibition in U1B, U4-U6 UsnRNAs expression. Apoptosis 11: 2267-2276.
- Hastak K, Agarwal MK, Mukhtar H, Agarwal ML (2005) Ablation of either p21 or Bax prevents p53-dependent apoptosis induced by green tea polyphenol epigallocatechin-3-gallate. FASEB J 19: 789-791.
- Soleas GJ, Goldberg DM, Grass L, Levesque M, Diamandis EP (2001) Do wine polyphenols modulate p53 gene expression in human cancer cell lines? Clin Biochem 34: 415-420.
- Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, et al. (1994) Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res 22: 3551-3555.
- Kaur M, Agarwal R, Agarwal C (2006) Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther 5: 1265-1274.
- Nakazato T, Ito K, Ikeda Y, Kizaki M (2005) Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin Cancer Res 11: 6040-6049.
- Wu PP, Kuo SC, Huang WW, Yang JS, Lai KC, et al. (2009) (-)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res 29: 1435-1442.
- Umeda D, Tachibana H, Yamada K (2005) Epigallocatechin-3-O-gallate disrupts stress fibers and the contractile ring by reducing myosin regulatory light chain phosphorylation mediated through the target molecule 67 kDa laminin receptor. Biochem Biophys Res Comm 333: 628-635.
- Hung CF, Huang TF, Chiang HS, Wu WB (2005) (-)-Epigallocatechin-3-gallate, a polyphenolic compound from green tea, inhibits fibroblast adhesion and migration through multiple mechanisms. J Cell Biochem 96: 183-197.
- Adams JC (2004) Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol 16: 590-596.
- Machesky LM, Li A (2010) Fascin: Invasive filopodia promoting metastasis. Commun Integr Biol 3: 263-270.
- Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, et al. (1985) Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res 44: 139-266.
- Lee MF, Pan MH, Chiou YS, Cheng AC, Huang H (2011) Resveratrol modulates MED28 (Magicin/EG-1) expression and inhibits epidermal growth factor (EGF)-induced migration in MDA-MB-231 human breast cancer cells. J Agric Food Chem 59: 11853-11861.
- Ko HS, Lee HJ, Kim SH, Lee EO (2012) Piceatannol suppresses breast cancer cell invasion through the inhibition of MMP-9: involvement of PI3K/AKT and NF-κB pathways. J Agric Food Chem 60: 4083-4089.
- Déziel BA, Patel K, Neto C, Gottschall-Pass K, Hurta RA (2010) Proanthocyanidins from the American Cranberry (Vaccinium macrocarpon) inhibit matrix metalloproteinase-2 and matrix metalloproteinase-9 activity in human prostate cancer cells via alterations in multiple cellular signalling pathways. J Cell Biochem 111: 742-754.
- Tate P, God J, Bibb R, Lu Q, Larcom LL (2004) Inhibition of metalloproteinase activity by fruit extracts. Cancer Lett 212: 153-158.
- Benelli R, Venè R, Bisacchi D, Garbisa S, Albini A (2002) Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem 383: 101-105.
- Kim S, Han J, Lee SK, Choi MY, Kim J, et al. (2012) Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-α in breast cancer cells. J Surg Res 176: e21-29.
- Kim M, Murakami A, Kawabata K, Ohigashi H (2005) (-)-Epigallocatechin-3-gallate promotes pro-matrix metalloproteinase-7 production via activation of the JNK1/2 pathway in HT-29 human colorectal cancer cells. Carcinogenesis 26: 1553-1562.
- Vaid M, Singh T, Katiyar SK (2011) Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS One 6: e21539.
- Madhan B, Subramanian V, Rao JR, Nair BU, Ramasami T (2005) Stabilization of collagen using plant polyphenol: role of catechin. Int J Biol Macromol 37: 47-53.
- Castillo-Pichardo L, Martínez-Montemayor MM, Martínez JE, Wall KM, Cubano LA, et al. (2009) Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin Exp Metastasis 26: 505-516.
- Goldberg DM, Hahn SE, Parkes JG (1995) Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chim Acta 237: 155-187.
- Huxley RR, Neil HA (2003) The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 57: 904-908.
- Friedman LA, Kimball AW (1986) Coronary heart disease mortality and alcohol consumption in Framingham. Am J Epidemiol 124: 481-489.
- Gollucke AP, Aguiar O Jr, Barbisan LF, Ribeiro DA (2013) Use of grape polyphenols against carcinogenesis: putative molecular mechanisms of action using in vitro and in vivo test systems. J Med Food 16: 199-205.
- Huang S, Yang N, Liu Y, Gao J, Huang T, et al. (2012) Grape seed proanthocyanidins inhibit colon cancer-induced angiogenesis through suppressing the expression of VEGF and Ang1. Int J Mol Med 30: 1410-1416.
- Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10: 175-176.
- Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48: 749-762.
- Yamakoshi J, Saito M, Kataoka S, Kikuchi M (2002) Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol 40: 599-607.
- Ray S, Bagchi D, Lim PM, Bagchi M, Gross SM, et al. (2001) Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol 109: 165-197.
- Shanmuganayagam D, Beahm MR, Osman HE, Krueger CG, Reed JD, et al. (2002) Grape seed and grape skin extracts elicit a greater antiplatelet effect when used in combination than when used individually in dogs and humans. J Nutr 132: 3592-3598.
- Violi F, Pignatelli P, Pulcinelli FM (2002) Synergism among flavonoids in inhibiting platelet aggregation and H2O2 production. Circulation 105: e53.
- Williams SN, Shih H, Guenette DK, Brackney W, Denison MS, et al. (2000) Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem Biol Interact 128: 211-229.
- Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, et al. (1999) Green tea and cancer chemoprevention. Mutat Res 428: 339-344.
- Loew D, Kaszkin M (2002) Approaching the problem of bioequivalence of herbal medicinal products. Phytother Res 16: 705-711.
- Billard C, Izard JC, Roman V, Kern C, Mathiot C, et al. (2002) Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk Lymphoma 43: 1991-2002.
- Eberhardt MV, Lee CY, Liu RH (2000) Antioxidant activity of fresh apples. Nature 405: 903-904.
- Temple NJ, Gladwin KK (2003) Fruit, vegetables, and the prevention of cancer: research challenges. Nutrition 19: 467-470.
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P (2000) Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 154-160.
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, et al. (1996) Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334: 1145-1149.
- Liu RH (2004) Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 134: 3479S-3485S.
- De Smet PA (2002) Herbal remedies. N Engl J Med 347: 2046-2056.